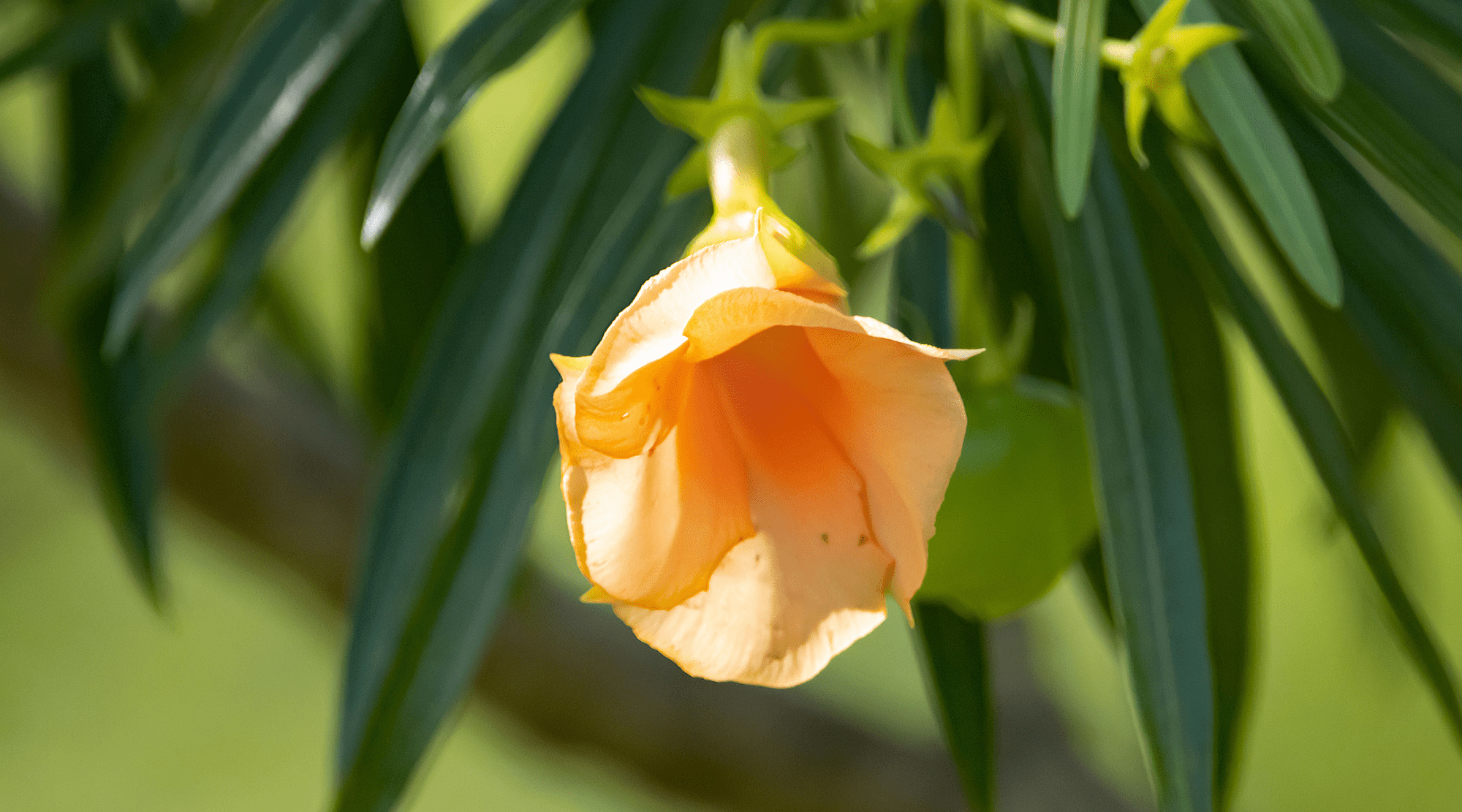
Since September, the FDA has been examining specific dietary supplements and has discovered instances where tejocote root products were contaminated with toxic yellow oleander, posing severe health risks and the potential for fatal reactions.
Many of these products are distributed online. As of April 4, the FDA has received reports of adverse effects from individuals who consumed products labelled as tejocote root. Notably, a serious incident has been linked to the Green ELV Nutrition brand Elv Control Herbal Supplement capsules, which have now been included on the FDA’s list of products containing toxic yellow oleander.
The FDA is cautioning consumers to steer clear of these products as they can lead to severe neurologic, gastrointestinal, and cardiovascular complications, some of which may be life-threatening. While several products have been recalled, seven suppliers have declined to recall their items, leaving the FDA concerned about the presence of toxic yellow oleander-laced products in the market. The FDA is exploring alternative regulatory measures to remove all identified products from circulation, although it has refrained from initiating mandatory recalls against the implicated companies.
The FDA possesses the authority to enforce recalls but has not yet exercised this power on the companies in question. A comprehensive list of the products is available in the table below.
Guidelines
- The FDA recommends that individuals discontinue using and properly dispose of these products.
- If you have used any of these concerning products, the FDA advises to contact your healthcare provider promptly. Even if the product hasn’t been used recently, inform your healthcare provider about the specific product for appropriate assessment.
- In case of serious side effects from these products, dial 9-1-1 or seek immediate medical assistance.
- If you or someone under your care has consumed these products recently and is experiencing health issues, contact your healthcare provider immediately.
- Consumers can reach out to the state poison control centre for further assistance.
Whom to Reach Out to
Healthcare professionals, patients, and consumers are urged to report complaints, exposure incidents, and adverse events to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
To report complaints or adverse events, such as illness or severe allergic reactions, you can:
- Contact an FDA Consumer Complaint Coordinator for personalized assistance with your concern.
- Fill out an online Electronic Voluntary MedWatch form.
- Complete a paper Voluntary MedWatch form and send it to the FDA.
Source: FSN
Reach out to Fresh Group Food Safety And Quality Consulting for any inquiries related to food quality and safety.